Oral Presentation Australian Diabetes Society and the Australian Diabetes Educators Association Annual Scientific Meeting 2014
Lixisenatide added to basal insulin reduces glycaemic variability in patients with type 2 diabetes mellitus (#96)
This analysis evaluated the impact on glycaemic variability (GV) of lixisenatide (LIXI) vs placebo (PBO) as add-on to basal insulin ± OADs in patients with T2DM
Patient-level data from GetGoal-L, GetGoal-Duo1, and GetGoal-L-Asia were pooled: standard deviation (SD); mean amplitude of glycaemic excursions (MAGE); mean absolute glucose (MAG); area under the curve-fasting glucose (AUC-F); and high and low blood glucose indices (HBGI/LBGI) were calculated. Changes in GV metrics over 24 weeks of treatment were compared. Relationships between baseline GV, patient characteristics, and clinical outcomes were assessed.
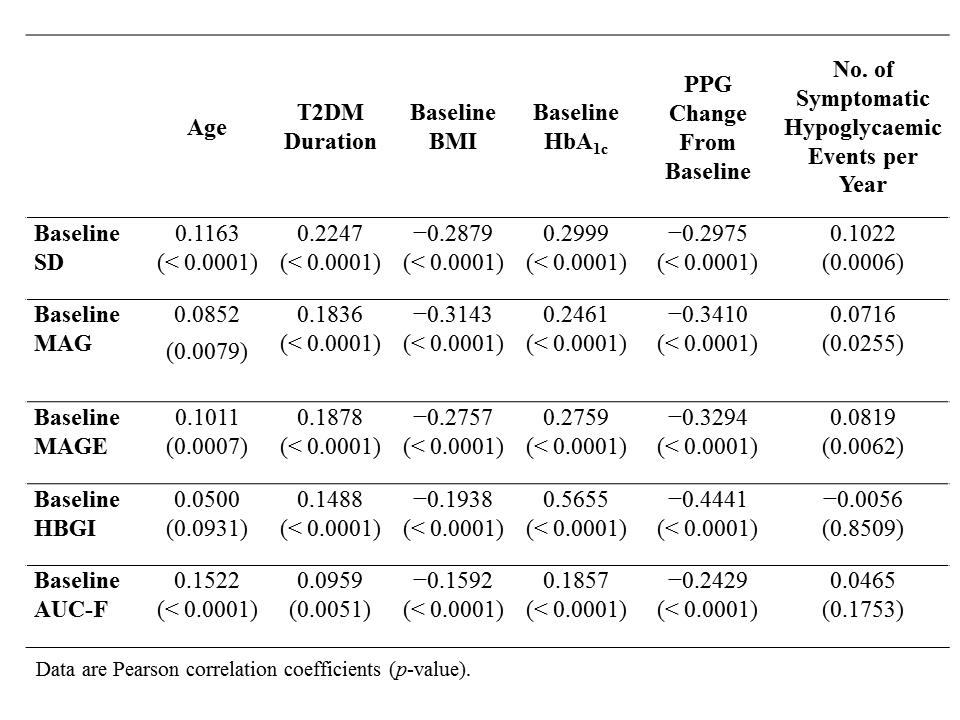
Data from 1,198 patients were analysed (665 LIXI and 533 PBO); 47.9% of patients were men, mean age was 57.2 years, mean BMI 30.4 kg/m2, mean T2DM duration 11.7 years, and mean HbA1c 8.1%. GV significantly decreased at Week 24 with LIXI vs PBO, respectively: SD −0.5 vs −0.2 mmol/L (p = 0.003); MAG −0.4 vs −0.1 mmol/L (p < 0.001); MAGE −0.9 vs −0.4 mmol/L (p = 0.003); HBGI −3.65 vs −0.88 (p < 0.001); and AUC-F −16.6 vs −0.9 mmolg/L*h (p < 0.001). LBGI was unchanged 0.04 vs −0.03 (p = 0.277). Higher baseline GV correlated with older age, longer T2DM duration, lower BMI, higher baseline HbA1c, greater postprandial plasma glucose (PPG) reduction, and higher symptomatic hypoglycaemia rates (Table).
Table. Relationship of baseline GV with patient characteristics.
When added to basal insulin ± OADs, LIXI significantly reduced GV and PPG excursions vs PBO, without increased risk of hypoglycaemia (as shown by LBGI). This may have relevance in patients with advanced T2DM where GV is increased..
Financial support: Study funding and editorial support provided by Sanofi US, Inc.