Poster Presentation Australian Diabetes Society and the Australian Diabetes Educators Association Annual Scientific Meeting 2014
Experience of three-times-daily biphasic insulin aspart in clinical practice: results from the A1chieve study (#335)
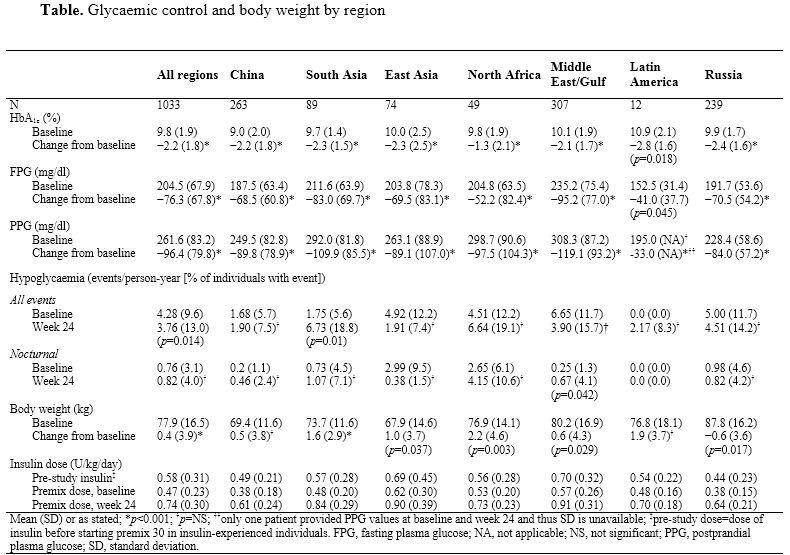
A1chieve was a non-interventional study evaluating the safety and clinical effectiveness of insulin analogues in people with type 2 diabetes (n=66 726) in routine clinical care in 28 countries across four continents. This A1chieve subgroup analysis included 1033 patients who received twice-daily (BID) biphasic insulin aspart 30 (premix 30) and were then intensified to three-times-daily (TID) premix 30 during the 24-week study period.
Mean age of the group was 56.4 (SD 12.2) years and diabetes duration 9.1 (6.6) years. At baseline, 833 (80.6%) of this subgroup were receiving oral glucose-lowering drugs. Glycaemic control was not to target at baseline (mean [SD] HbA1c and fasting plasma glucose were 9.8 [1.9] % and 204.5 [67.9] mg/dl, respectively). Both improved significantly following increased insulin dose/frequency, with significant reductions in post-breakfast plasma glucose level across the global regions being indicative of the mealtime effect of premix 30 (Table). Overall, a reduction in incidence of hypoglycaemia from 4.28 to 3.76 events/person-year was observed by 24 weeks. However, hypoglycaemia varied considerably by region. There was a small overall increase in nocturnal events associated with TID dosing. Change in body weight from baseline varied by region but was generally minimal, with an overall gain of 0.4 kg. In summary, this analysis confirms the efficacy and tolerability of an increase in premix 30 dosing frequency from BID to TID where required.
Acknowledgements: Novo Nordisk sponsored the A1chieve trial and abstract submission.