Oral Presentation Australian Diabetes Society and the Australian Diabetes Educators Association Annual Scientific Meeting 2014
How effective are GLP-1 agonists in real world practice - a multi centre study from the Australian S4S- DINGO - Diabetes Informatics Group (#98)
Background: The GLP-1 analogues, exenatide and liraglutide have recently become available in Australia for use in type 2 diabetes (T2DM), with purported benefits of improved glycaemic control and weight loss. However, there is a paucity of data on the efficacy, tolerability and safety of these incretin based therapies in Australian clinical practice.
Aim: To document the efficacy, tolerability and safety of GLP-1 analogues in real world practice.
Method: De-identified data of patients with T2DM were aggregated from the electronic medical record of endocrinologists using Audit4 (Software 4 Specialists, Australia & NZ). Data collected included demographics, start and cease dates of GLP-1 analogues, reason for cessation and serial HbA1c and weights, as well as all incident adverse events.
Results: From a cohort of 6,168 T2DM patients, 412 (6.7 %) had ever received a GLP-1 analogue, 85/15 % exenatide/liraglutide; compared to 48% of the cohort who had ever receive insulin. GLP-1 was commenced a mean 10.7 ± 6.8 y after diagnosis of diabetes when mean HbA1c and weight were 8.6 ±1.4 %, 103.5 ± 20.9 kg; and 24 months later HbA1c, weight had fallen to 7.7 ± 1.2 %, 99.6 ± 21.3 kg, respectively. 218 (46 %) of the GLP-1 treated patients ceased the drug a mean 7.6 months after initiation (range 0.2-41 months), 60% due to lack of efficacy and 30 % due to adverse effects mainly nausea. There were 3 incident episodes of pancreatitis, representing an overall incidence of 0.7 %.
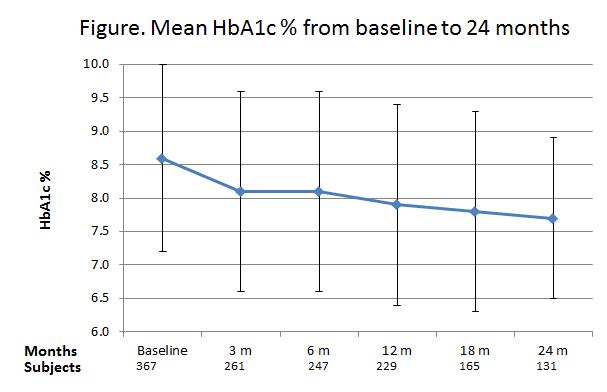
Summary: Our data indicate moderate efficacy of GLP-1 analogues with modest but progressive improvement in HbA1c consistent with clinical trials, but almost half the patients have stopped treatment by 8 months mostly due to lack of efficacy and adverse reactions. Acute pancreatitis is rare.
Conclusion: GLP-1 analogues have not been widely adopted by endocrinologists, which may relate to limitations of efficacy and tolerability.