Poster Presentation Australian Diabetes Society and the Australian Diabetes Educators Association Annual Scientific Meeting 2014
Real world effectiveness of vildagliptin vs other OADs as add-on to sulphonylureas in T2DM patients not receiving metformin: post-hoc analysis from the EDGE study (#316)
Guidelines recommend sulphonylureas (SUs) for first-line treatment in T2DM patients when metformin is contraindicated/not tolerated, when considered a preferred choice as per local guidelines or when baseline HbA1c is high. When treatment intensification is required after failing SU monotherapy, there is limited evidence behind recommendations for the optimal second-line choices. The aim of this post-hoc analysis of the multinational, observational EDGE study was to evaluate the effectiveness and safety of vildagliptin vs other oral anti-diabetic drugs (OADs) as add-on to first-line SUs (with no metformin at any time) in real-life settings.
Patients became eligible for inclusion in EDGE when an add-on OAD treatment was prescribed, based on clinical judgement, and were assigned to either vildagliptin or ‘other OADs’ (except incretin drugs) cohort. Primary endpoint was odds of achieving HbA1c reduction >0.3% without hypoglycaemia, weight gain ≥5%, peripheral oedema or discontinuation due to gastrointestinal side effects. Secondary endpoint was odds of achieving HbA1c <7.0% without hypoglycaemia or ≥3% weight gain in patients with baseline HbA1c >7.0%. HbA1c and weight change after 1 year were also evaluated.
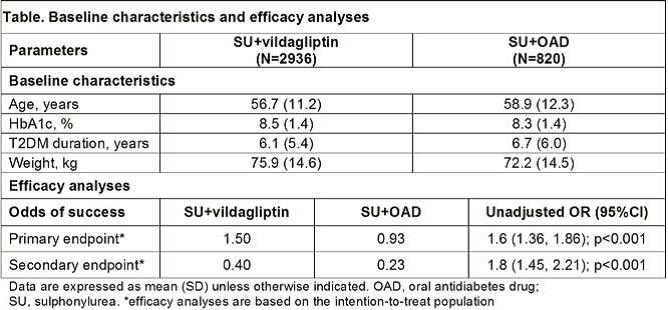
Of 3756 patients worldwide, 2936 received vildagliptin and 820 received other OADs, mostly thiazolidinediones (n=536), as add-on to SUs. Baseline characteristics were comparable between the cohorts (table). The odds of success for achieving the primary and secondary endpoints were in favour of vildagliptin (table). The adjusted mean (±SE) change in HbA1c from baseline were -1.4±0.02% and -1.2±0.03% in the vildagliptin and other OADs cohorts, respectively, with a between-treatment difference of -0.2±0.04% (p<0.0001). The change in body weight was -1.1 kg and -0.3 kg in the vildagliptin and other OADs cohort, respectively. Overall incidences of adverse events and hypoglycaemia were low in both cohorts.
In real-life settings, vildagliptin added to SU therapy led to significant improvement in glycaemia without weight gain, hypoglycaemia or other tolerability issues in patients unable to receive metformin.