Poster Presentation Australian Diabetes Society and the Australian Diabetes Educators Association Annual Scientific Meeting 2014
Glycaemic control in elderly people with T2DM in clinical trial, observational and individualised care settings (#294)
Treatment guidelines for elderly people with type 2 diabetes mellitus (T2DM) suggest less stringent, individualised treatment targets that take into account frailty status, comorbidities, polypharmacy, T2DM duration and T2DM-related complications. A literature review, however, reveals no supporting evidence and a scarcity of trials designed exclusively for these patients. Also, in elderly patients, it may be more appropriate to assess treatment outcomes using a composite endpoint (attainment of an individualised HbA1c target without hypoglycaemia or weight gain) rather than conventional uniform HbA1c targets. The aims of this post-hoc analysis were (i) to collect evidence from data sources representing diverse glycaemia management settings; and (ii) to assess the probability of success in reaching individualised and conventional glycaemic targets without tolerability issues in elderly T2DM patients treated with vildagliptin.
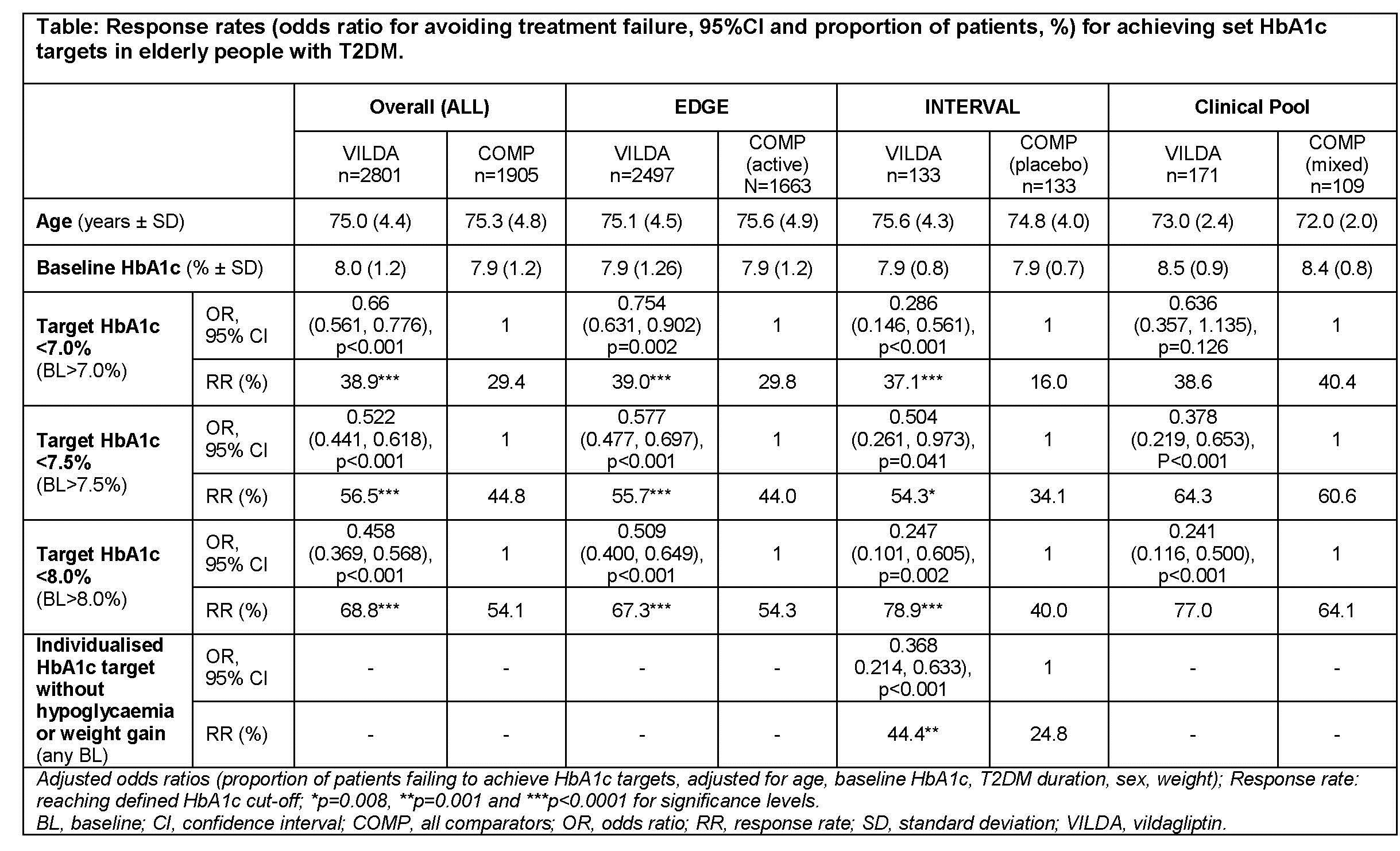
Using logistic regression models, we compared the efficacy of vildagliptin vs comparators (mostly sulphonylurea regimens), in achieving HbA1c targets of <7%, <7.5% and <8% in adults aged ≥70 years in 3 settings: conventional target-driven, pooled controlled clinical trials; a clinical trial using investigator-defined, individualised glycaemic targets in elderly, frail, multi-morbid T2DM patients (INTERVAL); and a real world, global, observational study (EDGE).
A representative population of 4706 patients was analysed; mean baseline (±SD) HbA1c was 8.0±1.2%, age 75.1±4.6 years and T2DM duration 9.0±6.9 years, balanced between data sets and treatment groups. In all 3 settings, vildagliptin had a significantly lower rate of treatment failure and was superior to comparators at achieving HbA1c targets (table). The odds of achieving individualised targets without hypoglycaemia or weight gain were also higher for vildagliptin (INTERVAL data shown).
Elderly patients treated with vildagliptin consistently achieved their glycaemic targets independent of the setting: clinical trials using conventional targets; a trial evaluating individualised targets in elderly patients; and a study in the real-world setting. Specific studies using more pragmatic outcomes in elderly T2DM patients, such as frailty and quality of life, remain to be performed.