Oral Presentation Australian Diabetes Society and the Australian Diabetes Educators Association Annual Scientific Meeting 2014
New Insulin Glargine 300 U/mL: Glycaemic Control and Hypoglycaemia in Insulin Naïve People with T2DM (EDITION 3) (#94)
EDITION 3 studied the efficacy and safety of new insulin glargine (300 U/mL; Gla-300) vs glargine 100 U/mL (Gla-100) in T2DM uncontrolled by treatments other than insulin.
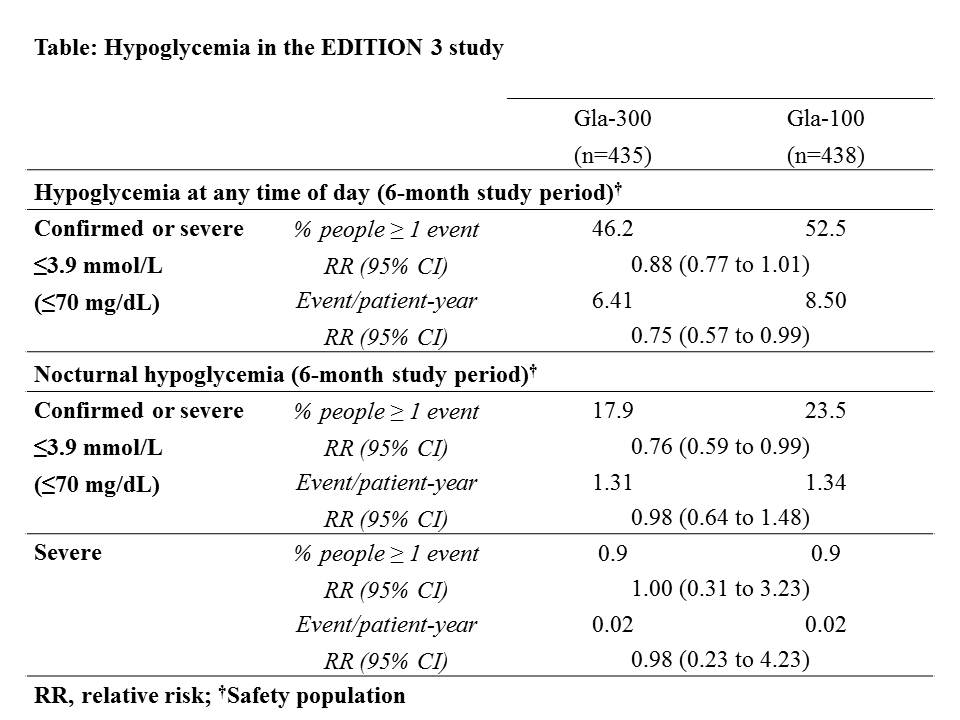
In this 6-month, multicenter, open-label study participants (n=878, BMI 33 kg/m2, T2DM duration 9.8 yr, HbA1c 8.5 %) were randomised to once-daily Gla-300 or Gla-100 in the evening while stopping sulfonylureas. Insulin was titrated seeking fasting self-measured plasma glucose of 4.4–5.6 mmol/L. Primary endpoint was HbA1c change to month 6, main secondary endpoint was percentage of participants with ≥1 confirmed (≤3.9 mmol/L [≤70 mg/dL]) or severe nocturnal hypoglycaemic event from week 9 to month 6.
HbA1c decreased similarly with both treatments (LS mean change [SD] -1.42 [0.05] and -1.46 [0.05] % difference 0.04 [95% CI: -0.09 to 0.17] %). RR of experiencing any confirmed or severe nocturnal hypoglycaemia with Gla-300 vs Gla-100 was 0.76 [CI: 0.59 to 0.99] for the 6 month treatment. Rates (per patient-yr) of confirmed or severe events at any time were lower with Gla-300 (RR 0.75 [CI: 0.57 to 0.99]) over 6 months. Severe hypoglycaemia was infrequent in both treatment groups. Mean weight change was +0.4 (SD 3.8) kg with Gla-300 and +0.7 (3.8) kg with Gla-100. No differences in adverse events were seen.
In conclusion, in insulin naïve people with T2DM, Gla-300 provides comparable effective glycaemic control with less hypoglycaemia vs Gla-100.
Funding: Study sponsored by sanofi (NCT01676220).